New Delhi, January 28:
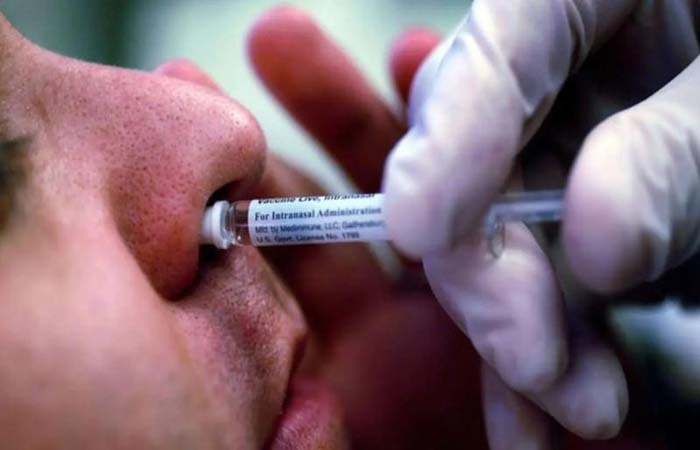
The Drugs Controller General of India (DCGI) on Friday gave its permission to Bharat Biotech for conducting trials of intranasal booster doses against the coronavirus disease (Covid-19).
According to an input from news agency ANI, the trials will be held at nice different sites.
A day ago, the Hyderabad-based pharmaceutical company’s anti-Covid vaccine Covaxin was recently granted regular market approval for use in the adult population subject to certain conditions. A similar nod has also been given to Serum Institute of India’s Covishield. Both the widely used vaccines in the country’s fight against the pandemic had so far been grante restricted use in emergency situations.